| Oxides Of Chlorine As Therapeutic Agents |
|---|
By Thomas L. Hesselink, MD of Aurora, Illinois
1st Edition - Copyright 2007 - All rights are reserved.
This
communication may not be reproduced in whole or in part
without written
permission from the author.
DISCLAIMERS:
- The information presented within these articles and accompanying communications is offered for legitimate educational and research purposes only.
- This exists as an exercise of the author's constitutional rights to freedom of speech and to freedom of the press.
- Nothing herein may be construed as providing:
medical advice, manufacturing advice, business advice, nor any advice whatsoever, nor endorsement of any kind. - No claims, no guarantees nor promises of suitability or efficacy for any purpose are made.
- The reader is further required and should agree not to aid nor to abet any deceptive, fraudulent, or illegal activity pertaining to any information obtained herein.
~~~ Links To Articles ~~~
| On The Mechanisms Of Toxicity Of Chlorine
Oxides Against Malarial Parasites - An Overview |
|---|
| Research Protocols And Precautions: |
| WWW References Pertaining To Oxides Of Chlorine: |
Bibliography Of Biologic And Therapeutic Oxidation
Dictionary Of Bio-Oxidative Medicine
Lectures In Hypertext - Home / Start Page
| On The Mechanisms Of Toxicity Of Chlorine Oxides Against Malarial Parasites - An Overview |
|---|
By Thomas Lee Hesselink, MD
Copyright September 6, 2007
- The purpose of this article is to propose research.
- Nothing in this article is intended as medical advice.
- No claims, promises nor guarantees are made.
ABSTRACT
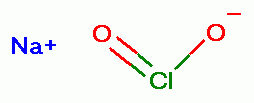 can be acidified as a
convenient method to produce chlorine dioxide (ClO2)
can be acidified as a
convenient method to produce chlorine dioxide (ClO2)  which is a strong oxidant
and a potent disinfectant. A protocol has been developed whereby a solution of
these compounds can be taken orally. This procedure rapidly eliminates malaria
and other infectious agents in only one dose. Chlorine dioxide (ClO2) is highly
reactive with thiols (RSH)
which is a strong oxidant
and a potent disinfectant. A protocol has been developed whereby a solution of
these compounds can be taken orally. This procedure rapidly eliminates malaria
and other infectious agents in only one dose. Chlorine dioxide (ClO2) is highly
reactive with thiols (RSH) 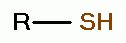 , polyamines
, polyamines 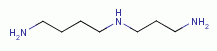 , purines
, purines 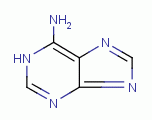 , certain amino acids and iron, all of which are
necessary for the growth and survival of pathogenic microbes. Properly dosed
this new treatment is tolerable orally with only transient side effects. More
research to better document efficacy in malaria and in other infections is
urgently called for.
, certain amino acids and iron, all of which are
necessary for the growth and survival of pathogenic microbes. Properly dosed
this new treatment is tolerable orally with only transient side effects. More
research to better document efficacy in malaria and in other infections is
urgently called for.
DISCOVERY
The procedure as used by Mr. Humble follows: A 28% stock solution of 80% (technical grade) sodium chlorite (NaClO2)
 is prepared. The remaining
20% is a mixture of the usual excipients necessary in the manufacture and
stabilization of sodium chlorite (NaClO2) powder or flake. Such are mostly
sodium chloride (NaCl) ~19%
is prepared. The remaining
20% is a mixture of the usual excipients necessary in the manufacture and
stabilization of sodium chlorite (NaClO2) powder or flake. Such are mostly
sodium chloride (NaCl) ~19% 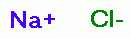 , sodium hydroxide (NaOH) <1%
, sodium hydroxide (NaOH) <1%  , and
sodium chlorate (NaClO3) <1%
, and
sodium chlorate (NaClO3) <1% 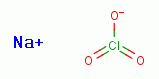 . The actual sodium chlorite (NaClO2)
present is therefore 22.4%. Using a medium caliber dropper (25 drops per cc),
the usual administered dose per treatment is 6 to 15 drops. In terms of
milligrams of sodium chlorite (NaClO2), this calculates out to 9mg per drop or
54mg to 135mg per treatment. Effectiveness is enhanced, if prior to
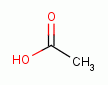
administration the selected drops are premixed with 2.5 to 5 cc of table vinegar
. The actual sodium chlorite (NaClO2)
present is therefore 22.4%. Using a medium caliber dropper (25 drops per cc),
the usual administered dose per treatment is 6 to 15 drops. In terms of
milligrams of sodium chlorite (NaClO2), this calculates out to 9mg per drop or
54mg to 135mg per treatment. Effectiveness is enhanced, if prior to
administration the selected drops are premixed with 2.5 to 5 cc of table vinegar
 or lime
juice or 5-10% citric acid
or lime
juice or 5-10% citric acid  and allowed to react for 3 minutes. The
resultant solution is always mixed into a glass of water or apple juice and
taken orally. The carboxylic acids neutralize the sodium hydroxide (NaOH)
and allowed to react for 3 minutes. The
resultant solution is always mixed into a glass of water or apple juice and
taken orally. The carboxylic acids neutralize the sodium hydroxide (NaOH)  and
at the same time convert a small portion of the chlorite (ClO2-)
and
at the same time convert a small portion of the chlorite (ClO2-)  to its
conjugate acid known as chlorous acid (HClO2)
to its
conjugate acid known as chlorous acid (HClO2) 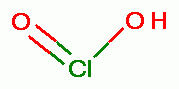 . Under such conditions the
chlorous acid (HClO2) will oxidize other chlorite anions (ClO2-)
. Under such conditions the
chlorous acid (HClO2) will oxidize other chlorite anions (ClO2-)  and
gradually produce chlorine dioxide (ClO2).
and
gradually produce chlorine dioxide (ClO2).  Chlorine dioxide (ClO2)
appears in solution as a yellow tint which smells exactly like elemental
chlorine (Cl2)
Chlorine dioxide (ClO2)
appears in solution as a yellow tint which smells exactly like elemental
chlorine (Cl2)  .
The above described procedure can be repeated a few hours later if necessary.
Considerably lower dosing should be applied in children or in emaciated
individuals scaled down according to size or weight. The diluted solution can be
taken without food to enhance effectiveness but this often causes nausea.
Drinking extra water usually relieves this. Nausea is less likely to occur if
food is present in the stomach. Starchy food is preferable to protein as protein
quenches chlorine dioxide (ClO2)
.
The above described procedure can be repeated a few hours later if necessary.
Considerably lower dosing should be applied in children or in emaciated
individuals scaled down according to size or weight. The diluted solution can be
taken without food to enhance effectiveness but this often causes nausea.
Drinking extra water usually relieves this. Nausea is less likely to occur if
food is present in the stomach. Starchy food is preferable to protein as protein
quenches chlorine dioxide (ClO2)  . Significant amounts of vitamin C
(ascorbic acid)
. Significant amounts of vitamin C
(ascorbic acid) 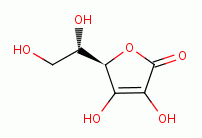 must not be present at any point in the
mixtures or else this will quench the chlorine dioxide (ClO2)
must not be present at any point in the
mixtures or else this will quench the chlorine dioxide (ClO2)  and
render it ineffective. For the same reason antioxidant supplements should not be
taken on the day of treatment. Other side effects reported are transient
vomiting, diarrhea, headache, dizziness, lethargy or malaise.
and
render it ineffective. For the same reason antioxidant supplements should not be
taken on the day of treatment. Other side effects reported are transient
vomiting, diarrhea, headache, dizziness, lethargy or malaise.
EXPLORING BENEFITS
 and chlorine dioxide (ClO2)
and chlorine dioxide (ClO2)  are
already widely used as disinfectants. What is novel and exciting here is that
Mr. Humble's technique seems: 1) easy to use, 2) rapidly acting, 3) successful,
4) apparently lacking in toxicity, and 5) affordable. If this treatment
continues to prove effective, it could be used to help rid the world of one of
the most devasting of all known plagues. Especially moving in me is the empathy
I feel for anyone with a debilitating febrile illness. I cannot forget how
horrible I feel whenever I have caught influenza. How much more miserable it
must be to suffer like that again and again every 2 to 3 days as happens in
malaria. Millions of people suffer this way year round. 1 to 3 million die from
malaria every year mostly children. Thus motivated I sought to learn all I could
about the chemistry of the oxides of chlorine. I wanted to understand their
probable mechanisms of toxicity towards the causative agents of malaria
(Plasmodium species). I wanted to check available literature pertaining to
issues of safety or risk in human use.
are
already widely used as disinfectants. What is novel and exciting here is that
Mr. Humble's technique seems: 1) easy to use, 2) rapidly acting, 3) successful,
4) apparently lacking in toxicity, and 5) affordable. If this treatment
continues to prove effective, it could be used to help rid the world of one of
the most devasting of all known plagues. Especially moving in me is the empathy
I feel for anyone with a debilitating febrile illness. I cannot forget how
horrible I feel whenever I have caught influenza. How much more miserable it
must be to suffer like that again and again every 2 to 3 days as happens in
malaria. Millions of people suffer this way year round. 1 to 3 million die from
malaria every year mostly children. Thus motivated I sought to learn all I could
about the chemistry of the oxides of chlorine. I wanted to understand their
probable mechanisms of toxicity towards the causative agents of malaria
(Plasmodium species). I wanted to check available literature pertaining to
issues of safety or risk in human use.
OXIDANTS AS PHYSIOLOGIC AGENTS
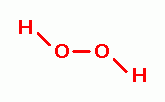 , zinc peroxide
, zinc peroxide 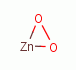 , various quinones (e.g.
benzoquinone
, various quinones (e.g.
benzoquinone 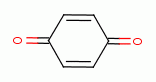 , rhodizonic acid)
, rhodizonic acid) 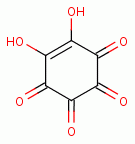 ,
various glyoxals (e.g. glyoxal
,
various glyoxals (e.g. glyoxal 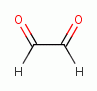 , methyl glyoxal
, methyl glyoxal 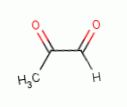 , ozone
, ozone  , ultraviolet light, hyperbaric oxygen
, benzoyl peroxide
, ultraviolet light, hyperbaric oxygen
, benzoyl peroxide  , anodes, artemisinin
, anodes, artemisinin  , methylene blue
, methylene blue
 ,
allicin
,
allicin 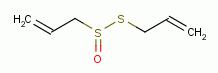 , iodine
, iodine
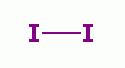 and permanganate
and permanganate 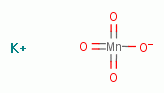 .
Some work has been done using dilute solutions of sodium chlorite (NaClO2)
.
Some work has been done using dilute solutions of sodium chlorite (NaClO2)  internally to treat fungal infections, chronic fatigue, and cancer; however,
little has been published in that regard.
internally to treat fungal infections, chronic fatigue, and cancer; however,
little has been published in that regard.
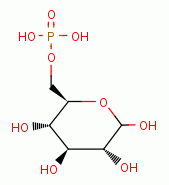
Low dose oxidant exposure to living red blood cells induces an increase in 2,3-diphosphoglycerate
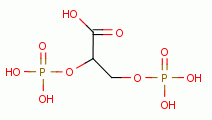 levels inside these cells. This
attaches to hemoglobin (Hb) in such a way that oxyhemoglobin (HbO2) more readily
releases oxygen (O2)
levels inside these cells. This
attaches to hemoglobin (Hb) in such a way that oxyhemoglobin (HbO2) more readily
releases oxygen (O2) 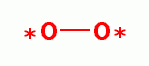 to the tissues throughout the body.
to the tissues throughout the body. Hyperbaric oxygenation (oxygen under pressure):
- is a powerful detoxifier against carbon monoxide;
- is a powerful support for natural healing in burns, crush injuries, and ischemic strokes; and
- is an effective aid to treat most bacterial infections.
Taken internally, intermittently and in low doses many oxidants have been found to be powerful immune stimulants. Sodium chlorite (NaClO2)
 acidified with lactic acid
acidified with lactic acid 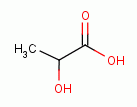 as in the product "WF10" has similarly been
shown to modulate immune activation. Exposure of live blood to ultraviolet light
also has immune enhancing effects. These treatments work through a natural
physiologic trigger mechanism, which induces peripheral white blood cells to
express and to release cytokines. These cytokines serve as a control system to
down-regulate allergic reactions and as an alarm system to increase cellular
attack against pathogens.
as in the product "WF10" has similarly been
shown to modulate immune activation. Exposure of live blood to ultraviolet light
also has immune enhancing effects. These treatments work through a natural
physiologic trigger mechanism, which induces peripheral white blood cells to
express and to release cytokines. These cytokines serve as a control system to
down-regulate allergic reactions and as an alarm system to increase cellular
attack against pathogens. The procedure as used by Mr. Humble follows: A 28% stock solution of 80% (technical grade) sodium chlorite (NaClO2)
 is prepared. The remaining
20% is a mixture of the usual excipients necessary in the manufacture and
stabilization of sodium chlorite (NaClO2) powder or flake. Such are mostly
sodium chloride (NaCl) ~19%
is prepared. The remaining
20% is a mixture of the usual excipients necessary in the manufacture and
stabilization of sodium chlorite (NaClO2) powder or flake. Such are mostly
sodium chloride (NaCl) ~19% 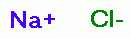 , sodium hydroxide (NaOH) <1%
, sodium hydroxide (NaOH) <1%  , and
sodium chlorate (NaClO3) <1%
, and
sodium chlorate (NaClO3) <1% 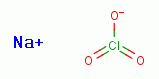 . The actual sodium chlorite (NaClO2)
present is therefore 22.4%. Using a medium caliber dropper (25 drops per cc),
the usual administered dose per treatment is 6 to 15 drops. In terms of
milligrams of sodium chlorite (NaClO2), this calculates out to 9mg per drop or
54mg to 135mg per treatment. Effectiveness is enhanced, if prior to
administration the selected drops are premixed with 2.5 to 5 cc of table vinegar
. The actual sodium chlorite (NaClO2)
present is therefore 22.4%. Using a medium caliber dropper (25 drops per cc),
the usual administered dose per treatment is 6 to 15 drops. In terms of
milligrams of sodium chlorite (NaClO2), this calculates out to 9mg per drop or
54mg to 135mg per treatment. Effectiveness is enhanced, if prior to
administration the selected drops are premixed with 2.5 to 5 cc of table vinegar
 or lime
juice or 5-10% citric acid
or lime
juice or 5-10% citric acid  and allowed to react for 3 minutes. The
resultant solution is always mixed into a glass of water or apple juice and
taken orally. The carboxylic acids neutralize the sodium hydroxide (NaOH)
and allowed to react for 3 minutes. The
resultant solution is always mixed into a glass of water or apple juice and
taken orally. The carboxylic acids neutralize the sodium hydroxide (NaOH)  and
at the same time convert a small portion of the chlorite (ClO2-)
and
at the same time convert a small portion of the chlorite (ClO2-)  to its
conjugate acid known as chlorous acid (HClO2)
to its
conjugate acid known as chlorous acid (HClO2)  . Under such conditions the
chlorous acid (HClO2) will oxidize other chlorite anions (ClO2-)
. Under such conditions the
chlorous acid (HClO2) will oxidize other chlorite anions (ClO2-)  and
gradually produce chlorine dioxide (ClO2).
and
gradually produce chlorine dioxide (ClO2).  Chlorine dioxide (ClO2)
appears in solution as a yellow tint which smells exactly like elemental
chlorine (Cl2)
Chlorine dioxide (ClO2)
appears in solution as a yellow tint which smells exactly like elemental
chlorine (Cl2)  .
The above described procedure can be repeated a few hours later if necessary.
Considerably lower dosing should be applied in children or in emaciated
individuals scaled down according to size or weight. The diluted solution can be
taken without food to enhance effectiveness but this often causes nausea.
Drinking extra water usually relieves this. Nausea is less likely to occur if
food is present in the stomach. Starchy food is preferable to protein as protein
quenches chlorine dioxide (ClO2)
.
The above described procedure can be repeated a few hours later if necessary.
Considerably lower dosing should be applied in children or in emaciated
individuals scaled down according to size or weight. The diluted solution can be
taken without food to enhance effectiveness but this often causes nausea.
Drinking extra water usually relieves this. Nausea is less likely to occur if
food is present in the stomach. Starchy food is preferable to protein as protein
quenches chlorine dioxide (ClO2)  . Significant amounts of vitamin C
(ascorbic acid)
. Significant amounts of vitamin C
(ascorbic acid)  must not be present at any point in the
mixtures or else this will quench the chlorine dioxide (ClO2)
must not be present at any point in the
mixtures or else this will quench the chlorine dioxide (ClO2)  and
render it ineffective. For the same reason antioxidant supplements should not be
taken on the day of treatment. Other side effects reported are transient
vomiting, diarrhea, headache, dizziness, lethargy or malaise.
and
render it ineffective. For the same reason antioxidant supplements should not be
taken on the day of treatment. Other side effects reported are transient
vomiting, diarrhea, headache, dizziness, lethargy or malaise.
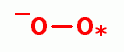 , hydrogen peroxide (H2O2)
, hydrogen peroxide (H2O2)  ,
hydroxyl radical (HO*)
,
hydroxyl radical (HO*)  , singlet oxygen (O=O)
, singlet oxygen (O=O) 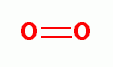 and ozone
(O3)
and ozone
(O3)  . Another is
peroxynitrate (-OONO)
. Another is
peroxynitrate (-OONO) 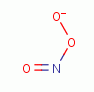 the coupled product of superoxide (*OO-)
the coupled product of superoxide (*OO-)
 and nitric
oxide (*NO)
and nitric
oxide (*NO)  radicals.
radicals.
| -OO* + *NO -> -OONO |
|---|
 the conjugate acid of
sodium hypochlorite (NaClO)
the conjugate acid of
sodium hypochlorite (NaClO)  . The immune system uses these
oxidants to attack various parasites.
. The immune system uses these
oxidants to attack various parasites.
OXIDES OF CHLORINE AS DISINFECTANTS
 are commonly used as bleaching agents, as swimming pool sanitizers, and as
disinfectants. At low concentrations chlorine dioxide (ClO2)
are commonly used as bleaching agents, as swimming pool sanitizers, and as
disinfectants. At low concentrations chlorine dioxide (ClO2)  has
been shown to kill many types of bacteria, viruses and protozoa. Ozone (O3)
has
been shown to kill many types of bacteria, viruses and protozoa. Ozone (O3)  or chlorine dioxide (ClO2)
or chlorine dioxide (ClO2)
 are often used to disinfect public water supplies or to sanitize and deodorize
waste water. Sodium chlorite (NaClO2)
are often used to disinfect public water supplies or to sanitize and deodorize
waste water. Sodium chlorite (NaClO2)  or chlorine dioxide (ClO2)
or chlorine dioxide (ClO2)  solutions are used in certain mouth washes to clear mouth odors and oral
bacteria. Chlorine dioxide (ClO2) sanitizes food preparation facilities.
Acidified sodium chlorite is FDA approved as a spray in the meat packing
industry to sanitized meat. This can also be used to sanitize vegetables and
other foods. Farmers use this to cleanse the udders of cows to prevent mastitis,
or to rid eggs of pathogenic bacteria. Chlorine dioxide (ClO2) can be used to
disinfect endoscopes. Oxidants such as iodine
solutions are used in certain mouth washes to clear mouth odors and oral
bacteria. Chlorine dioxide (ClO2) sanitizes food preparation facilities.
Acidified sodium chlorite is FDA approved as a spray in the meat packing
industry to sanitized meat. This can also be used to sanitize vegetables and
other foods. Farmers use this to cleanse the udders of cows to prevent mastitis,
or to rid eggs of pathogenic bacteria. Chlorine dioxide (ClO2) can be used to
disinfect endoscopes. Oxidants such as iodine 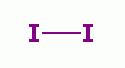 , various peroxides
, various peroxides  , permanganate
, permanganate  and chlorine dioxide
and chlorine dioxide  can be applied topically to the skin to
treat infections caused by bacteria or fungi.
can be applied topically to the skin to
treat infections caused by bacteria or fungi.
MALARIA IS OXIDANT SENSITIVE
 , artemether
, artemether
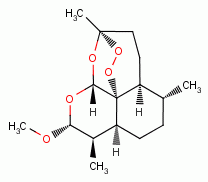 , t-butyl
hydroperoxide
, t-butyl
hydroperoxide 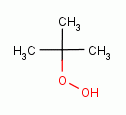 , xanthone
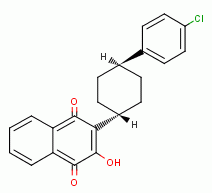
, xanthone 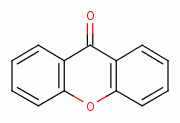 , various quinones (e.g. atovaquone
, various quinones (e.g. atovaquone
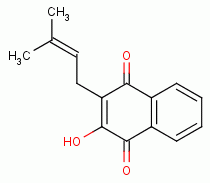
 , lapachol
, lapachol
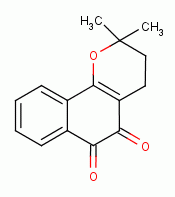
 , beta-lapachone
, beta-lapachone
 ,
menadione
,
menadione 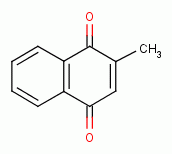 ) and
methylene blue
) and
methylene blue  .
.
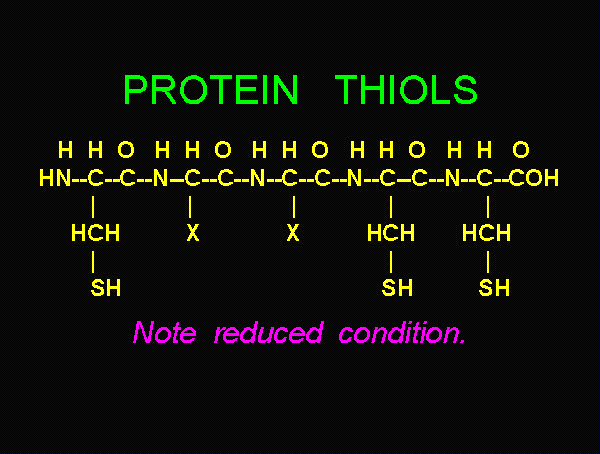
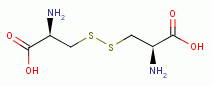
TARGETING THIOLS
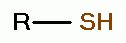 . Thiols as a class behave as reductants (electron
donors). As such they are especially sensitive to oxidants (electron grabbers).
. Thiols as a class behave as reductants (electron
donors). As such they are especially sensitive to oxidants (electron grabbers).
 and other sulfur compounds are reactive with sodium chlorite (NaClO2)
and other sulfur compounds are reactive with sodium chlorite (NaClO2)  and
with chlorine dioxide (ClO2)
and
with chlorine dioxide (ClO2)  . These are the very agents present in
Mr. Humble's solution. Possible products of oxidation of thiols (RSH)
. These are the very agents present in
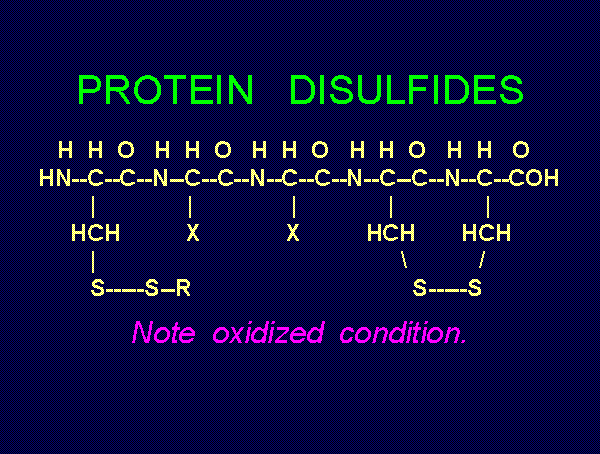
Mr. Humble's solution. Possible products of oxidation of thiols (RSH) 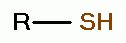 using various
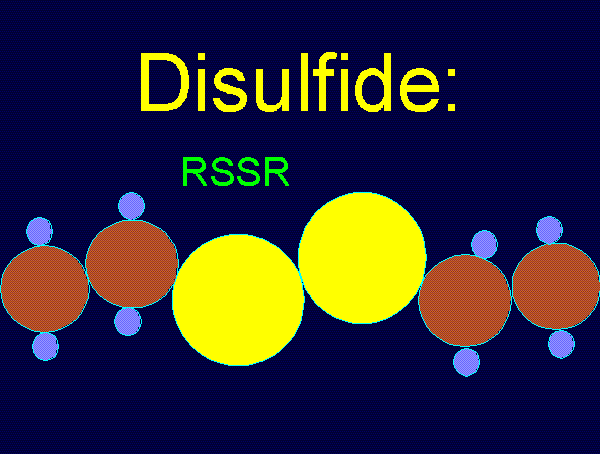
oxides of chlorine are: disulfides (RSSR)
using various
oxides of chlorine are: disulfides (RSSR) 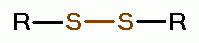 , disulfide monoxides (RSSOR)
, disulfide monoxides (RSSOR) 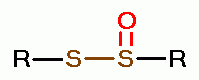 , sulfenic acids (RSOH)
, sulfenic acids (RSOH) 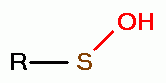 , sulfinic acids (RSO2H)
, sulfinic acids (RSO2H) 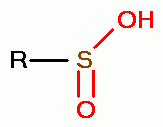 and
sulfonic acids (RSO3H)
and
sulfonic acids (RSO3H) 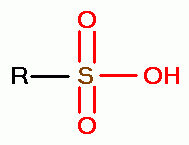 .
. 
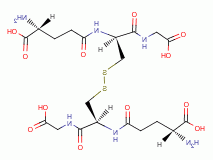
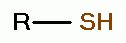 by oxidation, the parasite rapidly dies. A list of
thiols (RSH) upon which survival of Plasmodium species heavily depend includes:
dihydrolipoic acid
by oxidation, the parasite rapidly dies. A list of
thiols (RSH) upon which survival of Plasmodium species heavily depend includes:
dihydrolipoic acid  , coenzyme A
, coenzyme A 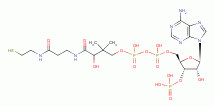 and acyl carrier protein
and acyl carrier protein 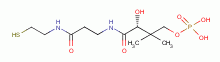 , glutathione
, glutathione  , glutathione reductase,
glutathione-S-transferase, peroxiredoxin, thioredoxin, glutaredoxin,
plasmoredoxin, thioredoxin reductase, falcipain and ornithine decarboxylase.
, glutathione reductase,
glutathione-S-transferase, peroxiredoxin, thioredoxin, glutaredoxin,
plasmoredoxin, thioredoxin reductase, falcipain and ornithine decarboxylase.
HEME IS AN OXIDANT SENSITIZER
 to
the more active chlorous acid (HClO2)
to
the more active chlorous acid (HClO2)  or chlorine dioxide (ClO2)
or chlorine dioxide (ClO2)  right
inside the parasite. Furthermore, Plasmodia consume 50 to 100 times more glucose
than noninfected red blood cells most of which is metabolized to lactic acid
right
inside the parasite. Furthermore, Plasmodia consume 50 to 100 times more glucose
than noninfected red blood cells most of which is metabolized to lactic acid
 another
known activator of chlorite (ClO2-).
Next falcipain a hemoglobin digesting enzyme hydrolyzes hemoglobin protein to
release its nutritional amino acids. A necessary byproduct of this digestion is
the release of 4 heme molecules
another
known activator of chlorite (ClO2-).
Next falcipain a hemoglobin digesting enzyme hydrolyzes hemoglobin protein to
release its nutritional amino acids. A necessary byproduct of this digestion is
the release of 4 heme molecules 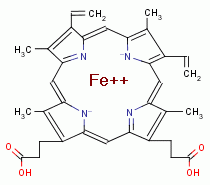 from each hemoglobin molecule digested. Free heme
(also known as ferriprotoporphyrin IX) is redox active and can react with
ambient oxygen (O2)
from each hemoglobin molecule digested. Free heme
(also known as ferriprotoporphyrin IX) is redox active and can react with
ambient oxygen (O2)  , an abundance of which is always present in red
blood cells. This produces superoxide radical (*OO-)
, an abundance of which is always present in red
blood cells. This produces superoxide radical (*OO-)  ,
hydrogen peroxide (H2O2)
,
hydrogen peroxide (H2O2)  and other reactive oxidant toxic species
(ROTS). These can rapidly poison the parasite internally. To protect themselves
against this dangerous side-effect of eating blood protein, Plasmodia must
maintain a high reductant capacity (an abundance of reduced thiols (RSH)
and other reactive oxidant toxic species
(ROTS). These can rapidly poison the parasite internally. To protect themselves
against this dangerous side-effect of eating blood protein, Plasmodia must
maintain a high reductant capacity (an abundance of reduced thiols (RSH)  and NADPH
and NADPH 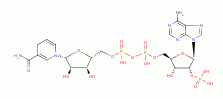 ) to quench these ROTS. This
is their main mechanism of antioxidant defense.
Plasmodia must also rapidly and continuously eliminate heme
) to quench these ROTS. This
is their main mechanism of antioxidant defense.
Plasmodia must also rapidly and continuously eliminate heme  , which is accomplished by two methods.
Firstly, heme is polymerized producing hemozoin. Secondly, heme is metabolized
in a detoxification process that requires reduced glutathione (GSH)
, which is accomplished by two methods.
Firstly, heme is polymerized producing hemozoin. Secondly, heme is metabolized
in a detoxification process that requires reduced glutathione (GSH)  . Therefore any
method (especially exposure to oxidants) which limits the availability of
reduced glutathione (GSH) will cause a toxic build up of heme and of ROTS inside
the parasite cells. Sodium chlorite (NaClO2) and chlorine dioxide (ClO2) (the
exact agents present in Mr. Humble's treatment) readily oxidize glutathione
(GSH). Therefore, a rapid killing of Plasmodia upon taking acidified sodium
chlorite orally should be expected.
. Therefore any
method (especially exposure to oxidants) which limits the availability of
reduced glutathione (GSH) will cause a toxic build up of heme and of ROTS inside
the parasite cells. Sodium chlorite (NaClO2) and chlorine dioxide (ClO2) (the
exact agents present in Mr. Humble's treatment) readily oxidize glutathione
(GSH). Therefore, a rapid killing of Plasmodia upon taking acidified sodium
chlorite orally should be expected.
OVERCOMING ANTIBIOTIC RESISTANCE WITH OXIDATION
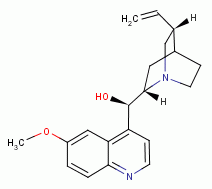 , chloroquine
, chloroquine 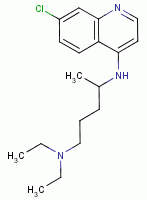 , mefloquine
, mefloquine 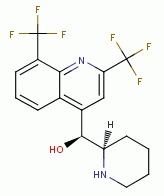 , quinacrine
, quinacrine  , amodiaquine
, amodiaquine  , primaquine
, primaquine  and other quinoline-like
antibiotics
and other quinoline-like
antibiotics  all
work by blocking the heme
all
work by blocking the heme  detoxifying system inside the trophozoites. Many Plasmodial strains against
which quinolines have repeatedly been used have found ways to adapt to these
drugs and to acquire resistance. Research into the mechanisms of resistance has
found that often resistance is accomplished by a meere upregulation of
glutathione (GSH)
detoxifying system inside the trophozoites. Many Plasmodial strains against
which quinolines have repeatedly been used have found ways to adapt to these
drugs and to acquire resistance. Research into the mechanisms of resistance has
found that often resistance is accomplished by a meere upregulation of
glutathione (GSH)  production and utilization. Consequently
oxidizing or otherwise depleting glutathione (GSH) inside the parasite usually
restores sensitivity to the quinoline antibiotics. Therefore, protocols
combining the use of oxidants with quinolines are under developement and already
showing signs of success. In this context let us consider that no amount of
intraplasmodial glutathione (GSH) could ever resist exposure to a suffient dose
of chlorine dioxide (ClO2)
production and utilization. Consequently
oxidizing or otherwise depleting glutathione (GSH) inside the parasite usually
restores sensitivity to the quinoline antibiotics. Therefore, protocols
combining the use of oxidants with quinolines are under developement and already
showing signs of success. In this context let us consider that no amount of
intraplasmodial glutathione (GSH) could ever resist exposure to a suffient dose
of chlorine dioxide (ClO2)  . Note that each molecule of chlorine
dioxide (ClO2) can disable 1 to 5 molecules of glutathione (GSH) depending on
the reaction mechanism.
. Note that each molecule of chlorine
dioxide (ClO2) can disable 1 to 5 molecules of glutathione (GSH) depending on
the reaction mechanism.
| 2(GSH) + 2(ClO2) --> 1(GSSG) + 2(H+) + 2(ClO2-)
or 10(GSH) + 2(ClO2) --> 5(GSSG) + 2(H+) + 2(Cl-) + 4(H2O) |
|---|
SOME INCOMPATIBILITIES
 by virtue of
its oxidative power. However, quinolines contain secondary amino groups
by virtue of
its oxidative power. However, quinolines contain secondary amino groups 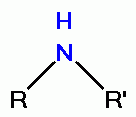 or tertiary amino groups
or tertiary amino groups 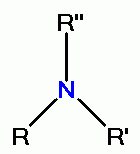 which react with chlorine dioxide (ClO2)
which react with chlorine dioxide (ClO2)
 in such a way that both could destroy each other. Some possible strategies to
resolve this incompatibility are suggested below.
in such a way that both could destroy each other. Some possible strategies to
resolve this incompatibility are suggested below.
- Acidified sodium chlorite could be used as explained above only as a solo therapy.
- Quinoline administration could be withheld until after the acidified sodium chorite has completed its action.
- Patients already preloaded with a quinoline could stop this, wait a suitable period of time for this to wash out, then administer the acidified sodium chlorite.
- The quinoline could remain in use and while the less active sodium chlorite is administered without acid. This should retain plenty of oxidant effectiveness without destroying any quinoline or wasting too much oxidant.
- Switch from a quinoline to an endoperoxide (such as artemisinin) or to a quinone (such as atovaquone) before using acidified sodium chlorite, as these may be less sensitive toward destruction by chlorine dioxide.
 and many other drugs if they have an
unoxidized sulfur atom, a phenol group
and many other drugs if they have an
unoxidized sulfur atom, a phenol group 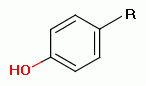 , a secondary amine
, a secondary amine  or a tertiary amine.
or a tertiary amine.  Such are reactive with the chlorine dioxide
(ClO2)
Such are reactive with the chlorine dioxide
(ClO2)  component.
component.
REDUCTANT RECOVERY SYSTEMS
 .
.
| 2 [H] + (RSSR) -> 2(RSH) |
|---|
 This enzyme is an
essential part of a complex process that produces NADPH
This enzyme is an
essential part of a complex process that produces NADPH  the main provider of reductants to the
reductases (enzymes which convert oxidized sulfur compounds back into thiols
(RSH)
the main provider of reductants to the
reductases (enzymes which convert oxidized sulfur compounds back into thiols
(RSH) 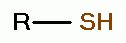 ).
Patients with a genetic defect of G6PDH, known as
glucose-6-phosphate-dehydrogenase deficiency disease, are especially sensitive
to oxidants and to prooxidant drugs. However, this genetic disease has a benefit
in that such individuals are naturally resistant to malaria. They can still
catch malaria, but it is much less severe in them, since they permanently lack
the enzyme necessary to assist the parasite in reactivating glutathione
disulfide (GSSG)
).
Patients with a genetic defect of G6PDH, known as
glucose-6-phosphate-dehydrogenase deficiency disease, are especially sensitive
to oxidants and to prooxidant drugs. However, this genetic disease has a benefit
in that such individuals are naturally resistant to malaria. They can still
catch malaria, but it is much less severe in them, since they permanently lack
the enzyme necessary to assist the parasite in reactivating glutathione
disulfide (GSSG)  and other oxidized thiols. Chlorine
dioxide (ClO2)
and other oxidized thiols. Chlorine
dioxide (ClO2)  has been shown to oxidize and denature
G6PDH by reaction with the tyrosine
has been shown to oxidize and denature
G6PDH by reaction with the tyrosine 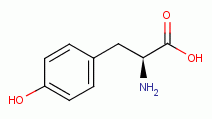 and the tryptophan
and the tryptophan 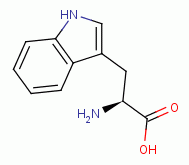 residues inside the enzyme.
Furthermore, G6PDH is sensitive to inhibition by sodium chlorate (NaClO3)
residues inside the enzyme.
Furthermore, G6PDH is sensitive to inhibition by sodium chlorate (NaClO3) 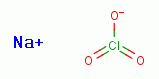 ,
another member of the chlorine oxide family of compounds. Sodium chlorate
(NaClO3) is a trace ingredient present in Jim Humble's antimalarial solution.
Some sodium chlorate (NaClO3)
,
another member of the chlorine oxide family of compounds. Sodium chlorate
(NaClO3) is a trace ingredient present in Jim Humble's antimalarial solution.
Some sodium chlorate (NaClO3) 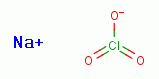 should also be produced in vivo by a slow
reaction of chlorine dioxide (ClO2) with water under alkaline conditions.
should also be produced in vivo by a slow
reaction of chlorine dioxide (ClO2) with water under alkaline conditions.
| 2(ClO2) + 2(OH-) -> (ClO2-) + (ClO3-) + H2O |
|---|
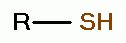 lost to
oxidation. However, this becomes more difficult as G6PDH is inhibited by
chlorine dioxide (ClO2)
lost to
oxidation. However, this becomes more difficult as G6PDH is inhibited by
chlorine dioxide (ClO2)  or by chlorate (ClO3-)
or by chlorate (ClO3-) 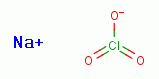 .
.
TARGETING IRON
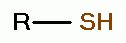 . Other
mechanisms of toxicity of the oxides of chlorine against Plasmodia should also
be considered. Oxides of chlorine are generally rapidly reactive with ferrous
iron (Fe++) converting it to ferric (Fe+++). This explains why in cases of
overdosed exposures to oxides of chlorine such as sodium chlorite (NaClO2)
. Other
mechanisms of toxicity of the oxides of chlorine against Plasmodia should also
be considered. Oxides of chlorine are generally rapidly reactive with ferrous
iron (Fe++) converting it to ferric (Fe+++). This explains why in cases of
overdosed exposures to oxides of chlorine such as sodium chlorite (NaClO2)  there
was a notable rise in methemoglobin levels. Methemoglobin is a metabolically
inactive form of hemoglobin in which its ferrous iron (Fe++) cofactor has been
oxidized to ferric (Fe+++).
there
was a notable rise in methemoglobin levels. Methemoglobin is a metabolically
inactive form of hemoglobin in which its ferrous iron (Fe++) cofactor has been
oxidized to ferric (Fe+++).  In living things including parasites iron is a
necessary cofactor for many enzymes. Thus it is reasonable to expect that any
damage to Plasmodia caused by oxides of chlorine is compounded by conversion of
ferrous (Fe++) cofactors to ferric (Fe+++) or other alterations of iron
compounds. Superoxide dismutase (SOD) inside Plasmodial cells also utilizes iron
in its active center. Chlorine dioxide
In living things including parasites iron is a
necessary cofactor for many enzymes. Thus it is reasonable to expect that any
damage to Plasmodia caused by oxides of chlorine is compounded by conversion of
ferrous (Fe++) cofactors to ferric (Fe+++) or other alterations of iron
compounds. Superoxide dismutase (SOD) inside Plasmodial cells also utilizes iron
in its active center. Chlorine dioxide  also oxidizes manganese (Mn++).
also oxidizes manganese (Mn++).
TARGETING POLYAMINES
 ,
which are deadly to parasites and to tumors. Chlorine dioxide (ClO2)
,
which are deadly to parasites and to tumors. Chlorine dioxide (ClO2)  is
known to be especially reactive against secondary amines
is
known to be especially reactive against secondary amines  . This includes spermine
. This includes spermine  and spermidine
and spermidine  the two main biologically
important polyamines.
the two main biologically
important polyamines. Thus any procedure, which is successful to oxidize both thiols (RSH)
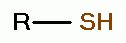 and polyamines does quadruple damage to the pathogen:
and polyamines does quadruple damage to the pathogen:
- oxidation of the thiol containing protein ornithine decarboxylase
inhibits polyamine synthesis; - oxidation of the thiol containing protein S-adenosyl-L-methionine
decarboxylase
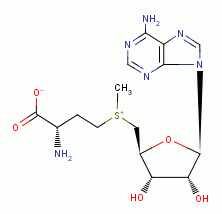
also inhibits polyamine synthesis; - oxidation of the secondary amines spermidine
 and spermine
and spermine 
depletes polyamine supplies; - the products of polyamine oxidation are toxic aldehydes
 .
.
TARGETING PURINES
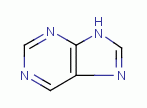 are essential to many life processes. These
molecules have a double ring structure. The rings are heterocyclic being
composed of both carbon and nitrogen. The secondary amino nitrogen atoms
are essential to many life processes. These
molecules have a double ring structure. The rings are heterocyclic being
composed of both carbon and nitrogen. The secondary amino nitrogen atoms  are vulnerable to reaction with chlorine
dioxide (ClO2)
are vulnerable to reaction with chlorine
dioxide (ClO2)  . Examples of important biologic purines
are xanthine
. Examples of important biologic purines
are xanthine 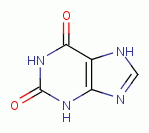 ,
hypoxanthine
,
hypoxanthine 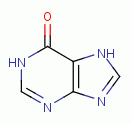 , inosine
, inosine 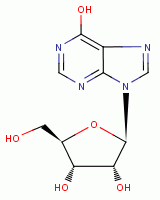 , guanine
, guanine 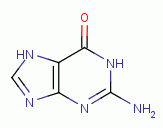 and adenine
and adenine  . Guanine and adenine are essential components of
DNA and RNA necessary for all genetic functions and for all protein syntheses.
Adenine is an essential component of the cofactors NADH
. Guanine and adenine are essential components of
DNA and RNA necessary for all genetic functions and for all protein syntheses.
Adenine is an essential component of the cofactors NADH 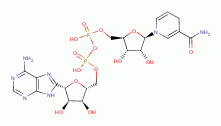 , NADPH
, NADPH  , FAD
, FAD 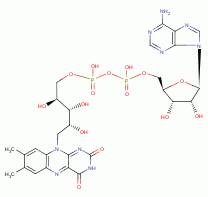 and ATP
and ATP 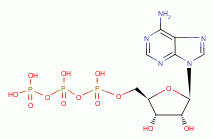 , necessary for many metabolic functions including
oxidation- reduction and energy metabolism. Any purines lost because of chlorine
dioxide (ClO2)
, necessary for many metabolic functions including
oxidation- reduction and energy metabolism. Any purines lost because of chlorine
dioxide (ClO2)  exposure can be readily replaced by host
cells. Plasmodia and other apicomplexae are uniquely vulnerable to purine
exposure can be readily replaced by host
cells. Plasmodia and other apicomplexae are uniquely vulnerable to purine  deficiency as they lack
the enzymes necessary to produce purines for themselves. Instead these must be
scavenged from host cells and imported across the plasma membranes of the
parasite cells. Drugs are under development to inhibit purine utilization by
Plasmodia and are already showing signs of success. Temporarily destroying some
of the purines in the blood as should occur upon brief exposure to chlorine
dioxide (ClO2)
deficiency as they lack
the enzymes necessary to produce purines for themselves. Instead these must be
scavenged from host cells and imported across the plasma membranes of the
parasite cells. Drugs are under development to inhibit purine utilization by
Plasmodia and are already showing signs of success. Temporarily destroying some
of the purines in the blood as should occur upon brief exposure to chlorine
dioxide (ClO2)  in vivo is probably an additional stress
that Plasmodia cannot tolerate.
in vivo is probably an additional stress
that Plasmodia cannot tolerate.
TARGETING PROTEINS
 is
highly reactive with thiols (RSH)
is
highly reactive with thiols (RSH) 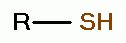 , phenols
, phenols  , secondary amines
, secondary amines  and tertiary amines
and tertiary amines  . Therefore, proteins composed of amino
acids which present these reactive groups are vulnerable to oxidation by this
agent. Proteins which present thiol groups as residue(s) of the amino acid
L-cysteine
. Therefore, proteins composed of amino
acids which present these reactive groups are vulnerable to oxidation by this
agent. Proteins which present thiol groups as residue(s) of the amino acid
L-cysteine 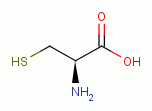 are discussed above under
TARGETING THIOLS. L-tyrosine
are discussed above under
TARGETING THIOLS. L-tyrosine  presents a phenol group and is therefore
similarly vulnerable. L-tryptophan
presents a phenol group and is therefore
similarly vulnerable. L-tryptophan  , L-histidine
, L-histidine 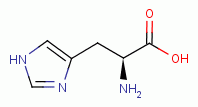 , L-proline
, L-proline 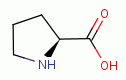 and 4-hydroy-L-proline
and 4-hydroy-L-proline 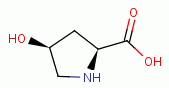 present secondary amino
groups which are also especially reactive with chlorine dioxide (ClO2)
present secondary amino
groups which are also especially reactive with chlorine dioxide (ClO2)  .
.
SAFETY ISSUES
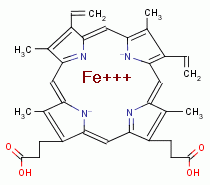 levels in frequent users. Sodium chlorite (NaClO2)
levels in frequent users. Sodium chlorite (NaClO2)  , as found in municipal water
supplies after disinfection by chorine dioxide (ClO2)
, as found in municipal water
supplies after disinfection by chorine dioxide (ClO2)  , has
been studied and proven safe. Animal studies using much higher oral or topical
doses have proven relatively safe. In a suicide attempt 10g of sodium chlorite
(NaClO2) taken orally caused refractory methemoglobinemia and nearly fatal
kidney failure. Inhalation or aerosol exposure to chlorine dioxide (ClO2) gas is
highly irritating and generally not recommended. Special precautions must be
employed in cases of glucose-6-phosphate-dehydrogenase deficiency disease, as
these patients are especially sensitive to oxidants of all kinds. Nevertheless,
oral acidified sodium chlorite solutions might even be found safe and effective
in them, but probably will need to be administered at lower doses.
, has
been studied and proven safe. Animal studies using much higher oral or topical
doses have proven relatively safe. In a suicide attempt 10g of sodium chlorite
(NaClO2) taken orally caused refractory methemoglobinemia and nearly fatal
kidney failure. Inhalation or aerosol exposure to chlorine dioxide (ClO2) gas is
highly irritating and generally not recommended. Special precautions must be
employed in cases of glucose-6-phosphate-dehydrogenase deficiency disease, as
these patients are especially sensitive to oxidants of all kinds. Nevertheless,
oral acidified sodium chlorite solutions might even be found safe and effective
in them, but probably will need to be administered at lower doses.
MORE RESEARCH
 dependent parasites should also be susceptible to
acidified sodium chlorite. For example Trypanosoma and Leishmania extensively
utilize and cannot survive without the cofactor known as trypanothione.
dependent parasites should also be susceptible to
acidified sodium chlorite. For example Trypanosoma and Leishmania extensively
utilize and cannot survive without the cofactor known as trypanothione. 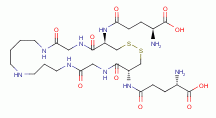 Each
molecule of trypanothione presents 2 sulfur atoms and 5 secondary amino groups
all of which are vulnerable to oxidative destruction from chlorine dioxide
(ClO2)
Each
molecule of trypanothione presents 2 sulfur atoms and 5 secondary amino groups
all of which are vulnerable to oxidative destruction from chlorine dioxide
(ClO2)  .
Chlorine dioxide (ClO2) has been proven to be cidal to almost all known
infectious agents in vitro using remarkably low concentrations. This includes
parasites, fungi, bacteria and viruses. The experiences noted above imply that
this compound is tolerable orally at effective concentrations. Therefore
extensive research is warranted to determine if acidified sodium chlorite is
effective in treating other infections. We may be on the verge of discovering
the most potent and broad spectrum antimicrobial agent yet known. Special thanks
go to Jim Humble for his willingness to share his discovery with the world.
.
Chlorine dioxide (ClO2) has been proven to be cidal to almost all known
infectious agents in vitro using remarkably low concentrations. This includes
parasites, fungi, bacteria and viruses. The experiences noted above imply that
this compound is tolerable orally at effective concentrations. Therefore
extensive research is warranted to determine if acidified sodium chlorite is
effective in treating other infections. We may be on the verge of discovering
the most potent and broad spectrum antimicrobial agent yet known. Special thanks
go to Jim Humble for his willingness to share his discovery with the world.
by Thomas Lee Hesselink, MD
References are available upon request.
Back to home page.
Die Oxidationsvorgänge im Zusammenhang mit der
Applikation von Chlordioxid
Vorwort:
Die folgenden Informationen beziehen sich auf den Bericht des amerikanischen Arztes MD Thomas Lee Hesselink
Der Bericht beruht auf der Analyse von 162 Literaturstellen. Anlass für seine Untersuchungen waren die Erfolge bei der Anwendung eines Produktes zur Erzeugung von Chlordioxid.
Anmerkung: Die „Ich-Form“ weist auf die Meinung von Thomas Lee Hesselink hin.
Es wird damit erstmalig eine Zusammenfassung der mit Chlordioxid im Zusammenhang stehenden Oxidationsvorgänge im Menschen gegeben. Mit diesen folgenden Beschreibungen können die in den WHO-Guidelines 2006 Werte zur toxischen Wirksamkeit von Chlordioxid hinsichtlich Bakterien, Viren und Parasiten plausibel begründet werden.
Besonders bemerkenswert sind Schlussfolgerungen von T.L. Hesselink bezüglich der Wirkung von Chloriten und Chloraten auf das Absterben von Parasiten.
Diese Darlegungen stehen im offensichtlichen Gegensatz zu der geläufigen Auffassung, dass Chlorite und Chlorate grundsätzlich für den menschlichen Organismus schädlich seien. Es scheint sich der Grundsatz „ Dosis facid venenum- Die Dosis macht das Gift“ zu bestätigen. Wenn Chlorite und Chlorate lediglich Gift für die Bakterien, Viren und Parasiten wären, dann ist das positiver und weiterhin untersuchungswürdiger Effekt.
Der Bericht kann all denen nützlich sein, die sich tiefere Klarheit über die ganzheitlichen desinfizierenden Wirkungen von Chlordioxid verschaffen wollen. Unabhängig von den Wirkungen von Chloridoxiden in den Stoffwechselprozessen der Lebewesen, werden durch Thomas Lee Hesseling wesentliche Argumente zur Anwendung von Chlordioxid für die Sicherung der Qualität des Wassers für den menschlichen Verzehr beschrieben. Es zeigt einen Weg zur Aufbereitung des Wassers für den menschlichen Verzehr in den subtropischen und tropischen Gebieten so zu behandeln.
Es folgen jetzt die zusammengefassten Erkenntnisse von Thomas Lee Hesselink:
1. Oxidationsmittel als physiologische Wirkstoffe
Mit den meisten anderen bekannten medizinisch nützlichen Oxidationsmitteln war ich bereits vertraut. Genannt seien hier:
Wasserstoffperoxid, Zinkperoxid, verschiedene Chinone, verschiedene Glyoxale, Ozon, ultraviolettes Licht, hyperbarer Sauerstoff, Benzylperoxid, Artemisinin, Methylenblau, Allizin, Jod, Permanganat.
Ich habe zahlreich Seminare darüber gehalten, wie man sie anwendet und wie sie auf biochemischer Ebene funktionieren.
Oxidationsmittel sind Atome oder Moleküle, die Elektronen aufnehmen. Reduktionsmittel dagegen sind Atome oder Moleküle, die Elektronen an Oxidationsmittel abgeben.
Setzt man lebende rote Blutkörperchen einer geringen Menge eines Oxidationsmittels aus, ändert sich die Oxyhämoglobin-Aktivität ( Hb-O2) dahingehend, dass mehr Sauerstoff in das Gewebe abgegeben wird. Hyperbare Oxygenierung ( unter erhöhten Druck gesetzter Sauerstoff):
- wirkt effektiv entgiftend gegen Kohlenstoffmonoxid;
- unterstützt die natürlichen Heilungsprozesse bei Verbrennungen, Quetschungen und ischämischen Schlaganfall immens und ist ein wirkungsvolles Mittel gegen bakterielle Infektionen.
- Innerlich, periodisch und in kleinen Dosen angewandt, stimulieren viele Oxidationsmittel das Immunsystem sehr effektiv. Eine ähnliche Wirkung erzielt man, wenn man lebende Blutzellen ultraviolettem Licht aussetzt.
Diese Behandlungsmethoden wirken durch einen natürlichen physiologischen Impulsmechanismus, der periphere weiße Blutkörperchen dazu anregt, Zytokine zu bilden. Diese Zytokine dienen als Alarmsystem und sorgen dafür, dass die Zellen vermehrt Krankheitserreger angreifen und allergische Reaktionen verhindert werden.
Aktivierte Zellen des Immunsystems produzieren im Rahmen eines Entzündungsprozesses wirksame natürliche Oxidationsmittel, um den Körper an Infektions- oder Krebsherden von der jeweiligen Krankheit zu befreien.
Eines dieser natürlichen Abwehr-Oxidationsmittel ist Wasserstoffperoxid. Ein anderes ist Peroxynitrat, ein Produkt aus Superoxid-Radikalen und Stickoxid-Radikalen. Ein weiterer ist hypochlorige Säure, die konjugierte Säure von Natriumhypochlorit.
2. Oxidationsmittel als Keimtöter
Verschiedene starke Oxidationsmittel sind als Desinfektionsmittel weit verbreitet. Es ist bewiesen, dass Bakterien nicht in der Lage sind, sich in einem Medium auszubreiten, das mehr ( Elektronen aufnehmende) Oxidationsmittel als ( Elektronen abgebende ) Reduktionsmittel enthält. Somit sind Oxidationsmittel bakteriostatisch, wenn nicht gar bakteriozid.
Einige Oxidationsmittel wie Jod, diverse Peroxide und Permanganat werden oberflächlich auf der Haut angewandt, um Infektionen vorzubeugen, die von Bakterien oder Pilzen ausgelöst werden. Chlordioxid wird ähnlich verwendet.
Hypochlorite kommen vor allem als Bleichmittel zum Einsatz, dienen aber auch zur Entkeimung von Schwimmbädern und als Desinfektionsmittel. Chlordioxid wie auch Ozon werden als keimtötende Mittel zur Wasseraufbereitung genutzt.
Natriumchlorit-Lösungen werden schon lange als Mundwasser gegen Mundgeruch und Bakterien im Mundraum verwendet. Mit Säure versetztes Natriumchlorit ist von der FDA in der Fleischverpackungsindustrie als Spray zur Desinfektion von Fleisch zugelassen. Landwirte benutzen das Mittel, um Kuheuter zu reinigen und so Mastitis vorzubeugen und um Eier von krankheitserregenden Bakterien zu reinigen. Chlordioxid tötet zudem viele Viren ab. Mit angesäuertem Natriumchlorit kann man sogar Gemüse keimfrei machen. Es wurden Versuche unternommen, mittel oral verabreichter Natriumchlorit-Lösungen Pilzinfektionen, chronische Müdigkeit und Krebs zu behandeln. In dieser Hinsicht ist leider nur sehr wenig in der Literatur veröffentlicht worden.
3. Malaria ist anfällig für Oxidationsmittel
Von November 2006 bis Mai 2007 habe ich viele 100 Stunden damit zugebracht, biochemische und medizinische Literatur nach der biochemischen Funktionsweise von Plasmodien zu durchsuchen. Vier Spezies sind für den menschlichen Körper pathogen:
- Plosmodium vivax
- Plasmodium falciparum
- Plosmodium ovale
- Plasmodium malariae.
Dabei stieß ich auf ein Fülle von hinweisen darauf, dass Plasmodien, ebenso wie Bakterien anfällig gegenüber Oxidationsmitteln sind.
Schädlich für Plasmodien sind beispielsweise: Artemisinin, Atovaquon, Menadion und Methylenblau.
Zudem hängt das Wachstum und Überleben von Plasmodien, wie auch von Bakterien und Tumorzellen, stark davon ab, ob genügend Thiol-Verbindungen vorhanden sind.
Thiole sind auch bekannt als Sulhydrylgruppen (RSH) und verhalten sich als Gruppe wie ( Elektronen abgebende ) Reduktionsmittel. Das macht sie höchst empfindlich gegenüber Oxidationsmitten. Sie reagieren leicht mit Chloroxiden und dazu zählen auch Natriunchlorit und Chlordioxid, eben die Wirkstoffe in Humbles Lösung.
Wenn Thiole mit Chloroxiden (so auch Chlordioxid) oxidieren, kommen u.a. folgende Stoffe heraus: Disulfid (RSSR), Disulfidmonooxid (RSSOR), Sulfensäure (RSOH), Sulfinsäure(RSO2H), Sulfonsäure (RSO3H). Alle diese Stoffe entziehen dem Parasiten die Lebensgrundlage.
Sind durch die Oxidation die für den Parasiten lebenswichtigen Thiole vernichtet, dann stirbt dieser.
Zu den für die Plasmodien-Spezies überlebensnotwendigen Thiolen gehören: Liponsäure und Dihydroliponsäure, das Coenzym A und das Acyl-Trägerprotein, Glutathion, Glutathion-Reduktase, Glutathionoxin, Plasmoredoxin, Thioredoxin-Reduktase,Ornithin-Decarboxylase, und Falcipain.
4. Häme als Sensibilisatoren von Oxidationsmitteln
Von besonderer Bedeutung für die Behandlung von Malaria ist, dass die bevorzugte Proteinquelle der plasmodialen Trophozoiten im Inneren der roten Blutkörperchen Hämoglobin ist. Dabei nehmen sie das Hämoglobin in eine Organelle auf, die sich „saure Nahrungsvakuole“ nennt. Die hohe Säurekonzentration in dieser Organelle könnte zusätzlich zur Umwandlung von Chlorit in das weit aktivere Chlordioxid beitragen, und zwar im Inneren des Parasiten.
Als nächstes hydrolisiert Falcipain ( ein Hämoglobin verdauendes Enzym) das Hämoglobin-Protein und setzt dessen nahrhafte Aminsäuren frei.
Ein wichtiges Nebenprodukt dieses Verdauungsprozesses ist, dass aus jedem verdauten Hämoglobin-Molekül vier Häm-Moleküle freigesetzt werden.
Freie Häm-Moleküle (auch Ferriprotoporphyrin genannt) sind redoxaktiv und können daher mit dem sie umgebenden Sauerstoff reagieren, von dem in roten Blutkörperchen immer reichlich vorhanden ist. Daraus entstehen Superoxid-Radikale, Wasserstoffperoxid und andere reaktive toxische Arten von Oxidationsmitten. Diese vergiften den Parasiten von innen heraus. Um sich selbst vor dem gefährlichen Nebeneffekt des Blutprotein-Konsums zu schützen, müssen Plasmodien permanent und schnell Häme eliminieren.
Das wird auf zwei Wegen erreicht: Zum einen werden Häm-Moleküle polymerisiert und bilden Hämozoin. Zum anderen werden Häme in einem Entgiftungsprozess abgebaut, für den reduziertes Glutathion (GSH) nötig ist.
Weil Herr Humble genau diese zwei Wirkstoffe in seinem Mittel verwendet, ist nur wahrscheinlich, dass der bereits beobachtete Effekt -das Absterben von Plasmodien- auch hier eintritt.
Daher sorgt alles, was verhindert, das der Parasit an reduziertes Glutathion gelangt( auch der Kontakt zu Oxidationsmitteln), in den Zellen des Parasiten für einen Stau an toxischen Häm-Molekülen. Da Natriumchlorit und Chlordioxid Glutathion oxidieren, unterbinden sie den Entgiftungsprozess des Parasiten.
5. Überwindung von Antibiotika-Resistenz mittels Oxidation
In diesem Zusammenhang muss auch die Resistenz der verschiedenen Plasmodien-Spezies gegenüber den gebräuchlichen antiprotozoalen Antibiotika angesprochen werden. Quinin, Chloroquin, Mefloquin und andere Chinolin-Antibiotika wirken dadurch, dass sie das Häm-Entgiftungssystem in den Trophozoiten blockieren. Viele Plasmodien-Stämme, gegen die wiederholt mit Chinolin vorgegangen wurde, haben einen Weg gefunden sich anzupassen und eine Resistenz zu entwickeln. Jüngste Forschungsarbeiten haben jedoch gezeigt, dass der Mechanismus hinter der entwickelten Resistenz, lediglich darin besteht, dass vermehrt Glutathion produziert und eingesetzt wird. Diese Arbeiten haben ebenfalls nachgewiesen, dass die Parasiten wieder auf Quinolin-Antibiotika ansprechen, sobald das Glutathion oxidiert oder anderweitig vermindert wird. Einige Versuche, bei denen man Oxidationsmittel parallel zu Quinolinen eingesetzt hat, haben bereits erste Erfolge gezeitigt. Hierbei gilt es zu berücksichtigen, dass keine noch so große Menge an Glutathion (GSH) in den Plasmodien je gegen eine genügend hohe Dosis Chlordioxid bestehen könnte. Man beachte, dass jedes Chlordioxid-Molekül je 5 Glutathion-Moleküle unschädlich machen kann.
Lebewesen besitzen ein Wiedergewinnungssystem, durch das sie oxidierte Schwefelverbindungen wiedergewinnen können. Dabei werden Wasserstoffatome an diese Schwefel-Verbindungen abgegeben und so deren ursprünglicher Zustand als Thiole wiederhergestellt.
Das Enzym Glukose-6-Phosphat-Dehydrogenase (G6PDH) spielt hierbei eine Schlüsselrolle. Patienten mit einem genetisch bedingten G6PDH-Defekt, besser bekannt als Glukose-6-Phosphat-Dehydrogenase-Mangel, reagieren besonders empfindlich auf Oxidationsmittel und prooxidative Medikamente. Diese genetisch bedingte Erkrankung hat allerdings den Vorteil, dass die Betroffenen gemeinhin resistent gegen Malaria sind. Zwar können auch sie erkranken, doch verläuft die Erkrankung bei ihnen weit weniger schlimm, da sie nicht über eine genügende Menge an dem Enzym verfügen, dass der Parasit braucht, um das Glutathion zu reaktivieren.
Zudem wird G6PDH leicht durch Natriumchlorat gehemmt, ein weiteres Mitglied der Familie der Chloroxid-Verbindungen. Natriumchlorat ist in geringem Maße auch in Jim Humbles Anti-Malaria-Lösung enthalten. Unter leicht alkalischen Bedingungen dürfte sich ein wenig Natriumchlorat auch in vivo als Folge der langsamen Reaktion von Chlordioxid und Wasser bilden. Die Plasmodien versuchen zwar, das Glutathion zu ersetzen, das bei der Oxidation verloren geht. Wenn G6PDH aber durch Chlorat gehemmt wird, ist dies sehr schwer, wenn nicht gar unmöglich.
6. Die Bedeutung von Eisen
Obgleich ein Grossteil der verfügbaren Literatur sich allein auf das gestörte Redox-Gleichgewicht bezieht, das zu einem Mangel an Thiolen führt, sollten auch andere toxische Mechanismen berücksichtigt werden, mit denen Chloroxid gegen Plasmodien vorgeht. Chloroxide (z.B.:Chlorite) reagieren für gewöhnlich mit zweiwertigem Eisen. Das erklärt, warum es beim Kontakt zu großen Mengen Chloroxiden, wie z.B. Natriumchlorit, zu einem deutlichen Anstieg des Methämoglobin- Spiegels kommt. Methämoglobin ist eine den Stoffwechsel nicht beeinflussende Form des Hämoglobins, in der der Cofaktor zweiwertiges Eisen zu dreiwertigem Eisen oxidiert ist. Viele Enzyme in Lebewesen, auch in Parasiten, verwenden Eisen als Cofaktor. Daher ist anzunehmen, dass jede Schädigung der Plasmodien durch Chloroxide (Chlorite, Chlorate) von der Wandlung der zweiwertigen zu dreiwertigen Eisen-Cofaktoren begleitet wird.
7. Die Bedeutung der Polyamine
Weitere Metaboliten, die Tumore, Bakterien und Parasiten zum Wachstum und Überleben brauchen, sind Polyamine. Fehlen diese, können Erreger nicht mehr wachsen, sie sterben. Auch Polyamine reagieren leicht auf Oxidation und lassen sich durch starke Oxidationsmittel zerstören.
Wenn Polyamine oxidieren, verwandeln sie sich in Aldehyde, die wiederum tödlich für Parasiten und Tumorzellen sind.
Somit richtet alles, was Polyamine oxidiert, doppelten Schaden an den Krankheitserregern an. Man weiss, dass gerade Chlordioxid besonders heftig mit sekundären Aminen reagiert. Zu den sekundären Aminen zählen auch Spermien und Spermiden, die aus biologischer Sicht beiden wichtigsten Polyaminen.
Quelle:
1) Jim Humble ; MMS-Der Durchbruch; Deutsche Erstausgabe 2008,Mobiwell-Verlag, Potsdam 2008, Anhang 1, S.186-204)
2) http://www.healthsalon.org/309/thomas-lee-hesselink-md-writes-on-mms-and-sodium-chlorite/
3) http://www.npi.gov.au/database/substance-info/profiles/21.html
Anmerkung:
Nach meinen weltweiten Recherchen kann der Bericht von Thomas Lee Hesseling zu den Oxidationsvorgängen im Zusammenhang mit Chlordioxid als besonders wertvoll eingestuft werden. Er stützt die Ausführungen in dem Arbeitsblatt „ Informationen zu Haftungsrisiken bei der Trinkwasserversorgung“ und vertieft die Applikation von Chlordioxid im allgemeinen und im besonderen zur mikrobiologisch unbedenklichen Herstellung von Wasser für den menschlichen Verzehr. Damit ist Chlordioxid auch in der Getränkeindustrie ( z.B: Coca-Cola) ein zu favorisierendes Mittel zur Desinfektion der Anlagen und Geräte und des durchfließendes Wassers, das für den menschlichen Gebrauch bestimmt ist.
(Dr.-Ing. Wolfgang Storch)
Mai 2009